HDG process
Hot-dip galvanizing is a method of immersion which means that the whole process from surface preparation to zinc coating is conducted by dipping steel elements in kettles containing suitable chemical baths.
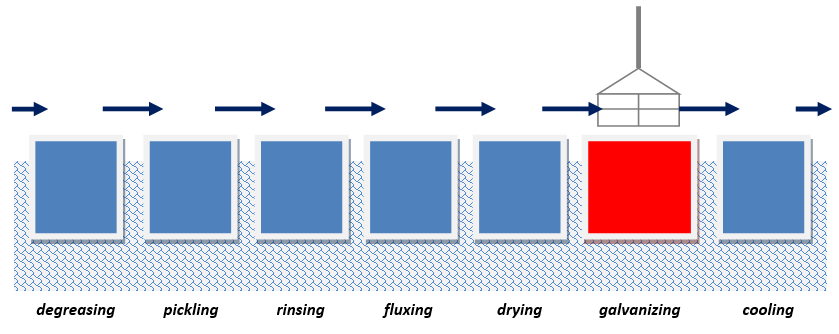
Degreasing
It is the first step of preparation which aims at eliminating organic contaminants (grease, wax substances, oils) which may make the reaction between steel and zinc impossible. The aim of this process is to obtain chemically-clean surfaces through the use of non-organic solutions such as acidic, alkali or neutral.
Pickling
This stage aims at removing non-metalic substances from the surface (primarily rust, mill scale and other iron oxides). The most common way of pickling is using the solution of hydrochloric acid (HCl). If the pickling process has been successful, the surface will be clean and devoid of iron oxides.
Rinsing
Rinsing prevents excessive transfer of technological baths to further stages of the chemical treatment and it also prolongs the effectiveness of each technological bath.
Fluxing
During this step, pickled steel is immersed in aqueous solution of zinc chloride and ammonium chloride. Flux removes any remaining oxides and ensures proper chemical reactions during the hot-dip galvanizing prosess.
Drying
After fluxing the steel elements are dried until 120°C – 150°C. The aim of this process is to remove excess amounts of flux and to heat the steel to the right temperature in order to minimize thermal shock while immersing the steel in the zinc bath.
Hot-dip galvanizing
During the true galvanizing stage of the process, the material is immersed in a molten zinc bath (approximately 450°C). The final step of the process is to form a tightly-bonded alloy zinc coating which results from the reaction of zinc and iron, also called defusion, that is when atoms of zinc bond with outer steel layers.
Cooling
Cooling is the last step of the galvanizing process which aims at hardening and cooling galvanized articles to room temperature.
Properly galvanized coating protects steel for years to come depending on environmental conditions. Hot-dip galvanized constitutes both physical and electrolyte protection, which results from the standard potential of zinc being much more negative than that of iron. Therefore, zinc will corrode first and its corrosion effects will slow down further steel surface rusting. Even if zinc coating is dameged, for example mechanically, it will still protect the goods from corrosion.

